While the recently announced ‘new rules’ setting out conditionalities for the importation of drugs into Guyana, including the stipulation that the labels be printed in English may be well-intentioned, they are likely to have very little effect unless the Government Analyst-Food & Drug Department (GAFDD) is given “both the authority and the tools” to ensure that the news rules are “rigidly enforced, an experienced local Hospital Administrator has told the Stabroek Business.
Last week the GAFDD announced that all drugs imported into Guyana must be registered with the Department in compliance with a New Drug Registration requirement, Food and Drug Act Chap 34:03-Regulations 1977, Regulation 78. Additionally, the GAFDD stipulated in its release that “the brand and generic name, the address of the manufacturer and the patient information leaflet must be stated in English language on all finished product packages.” The requirement is attended by a threat that all drugs (prescription and over the counter) that are labelled in a foreign language will be seized and removed from premises.”
But the source told Stabroek Business that “at least up to this time” the declaration by the GAFDD “amounts to no more than huffing and puffing,” a condition which he says will remain unchanged “until it has the authority and the resources” to enforce those stipulations.
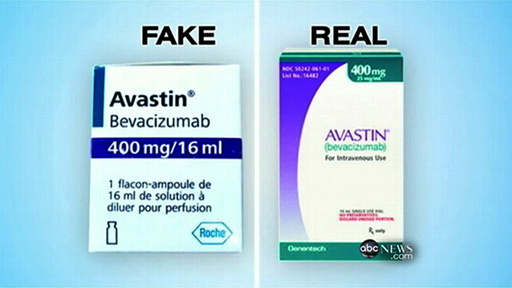 Charging that there are “numerous instances” in which the GAFDD is “none the wiser” regarding whether or not the drugs imported into Guyana are “fake or otherwise,” the source said that the problem facing the Department is that “its mandate extends way beyond its capabilities…It really makes little sense to lay down stipulations and threaten sanctions if you do not have the reach to access much of the drugs that are coming in under the radar and which really ought to be the target of your operations.” “What I am saying,” he added, “is that the Food and Drug Department has to be given sturdier tools besides the public statements that they make if their enforcement powers are to be respected. The reality is that the Department is not ideally equipped to make good the threats that they make about seizures and other forms of sanction because, as far as I am aware they have neither the manpower nor the authority to do what needs to be done.”
Charging that there are “numerous instances” in which the GAFDD is “none the wiser” regarding whether or not the drugs imported into Guyana are “fake or otherwise,” the source said that the problem facing the Department is that “its mandate extends way beyond its capabilities…It really makes little sense to lay down stipulations and threaten sanctions if you do not have the reach to access much of the drugs that are coming in under the radar and which really ought to be the target of your operations.” “What I am saying,” he added, “is that the Food and Drug Department has to be given sturdier tools besides the public statements that they make if their enforcement powers are to be respected. The reality is that the Department is not ideally equipped to make good the threats that they make about seizures and other forms of sanction because, as far as I am aware they have neither the manpower nor the authority to do what needs to be done.”
There are, according to the source, two considerations that impact negatively on the ability of the Food and Drug Department to carry out its threats. “The first is the complete failure by government over the years to incrementally build the capacity of Food and Drugs to effectively execute what has been its increasingly important role. The second has to do with the fact that in a situation where the global fake drugs industry is valued at billions of dollars, considerations of bribery and corruption have arisen here at the local level and there is a widespread view that it goes high up. This makes the job of an enforcement agency like Food and Drugs difficult.
Current Director of the GAFDD, Marlan Cole, has repeatedly commented on the human resource limitations confronting the Department, a circumstance which he says inhibits its ability to perform its inspection functions effectively.
Numerous cases have arisen in which public officials have been accused of accepting kickbacks from importers to allow for the clandestine importation of drugs that do not comply with official regulations. Some of these drugs, it is believed, find their way to a number of state-run and private medical institutions across the country.
While the new regulations stipulate that all drugs imported into Guyana must be registered with the GAFDD “in compliance with a New Drug Registration requirement, Food and Drug Act Chap 34:03-Regulations 1977, Regulation 78,” the Stabroek Business source said that such a stipulation can only be enforced “if the Department has a clear idea regarding all of the entry points through which drugs enter the country.” “You would be surprised if you were to come to a full understanding of all of the various ways through which drugs enter the country,” the source said.
Dripping with corruption, the global fake drugs industry is estimated to have penetrated poor countries to the extent that one in 10 medicines imported into those countries are counterfeit and likely responsible for the deaths of tens of thousands of children and adults from diseases ranging from malaria and pneumonia to cancer, every year.
Counterfeit drugs include products that have not been approved by regulators, fail to meet quality standards or deliberately misrepresent an ingredient, according to the WHO.
In 2013, the WHO set up a voluntary global monitoring system for substandard and fake drugs and has received reports of about 1,500 problematic medicines including drugs that claim to treat heart problems, diabetes, fertility problems, mental health issues and cancer. Some of these drugs reportedly find their way into state hospitals where the overwhelming majority of patients are working class people.
The WHO estimates that several countries could be spending in excess of US$23 billion on counterfeit drugs.
“Once you examine all of the dimensions to the fake drugs industry as uncovered by the WHO and other research entities you very quickly realise just how helpless our own Food and Drug Department is in, given its present state,” the source said.






