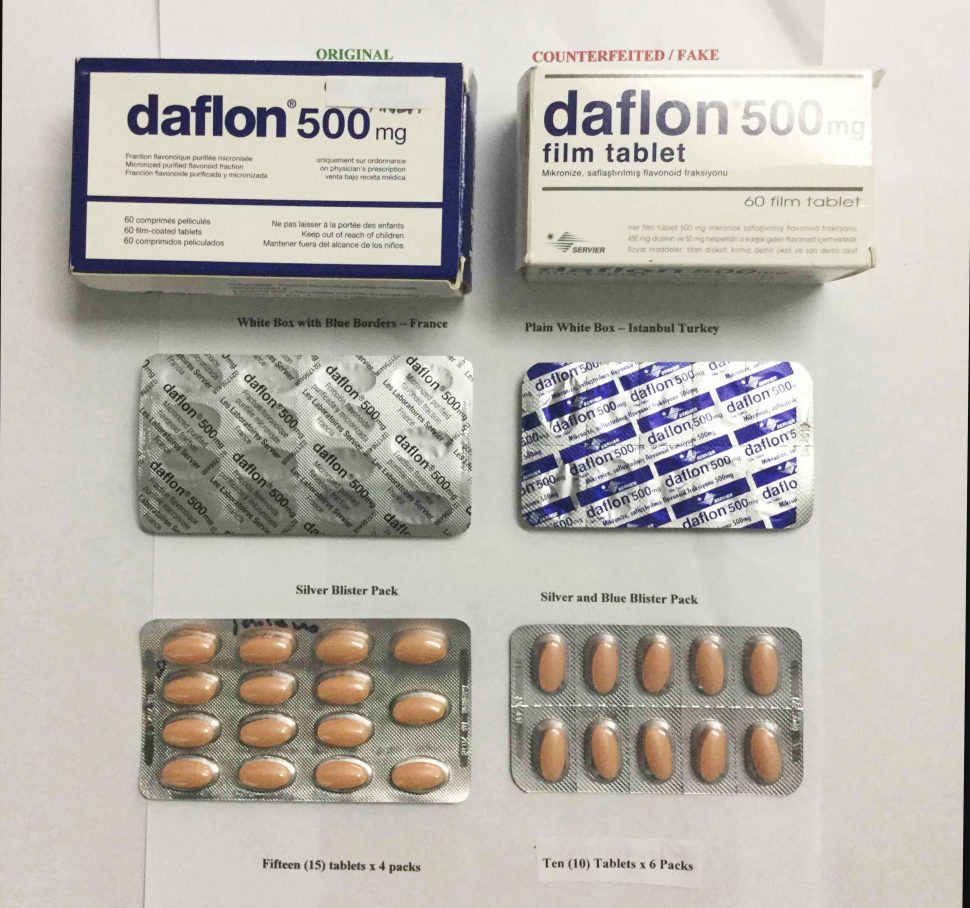
The Government Analyst–Food and Drug Department (GA-FDD) is notifying the general public about the circulation of a fake/counterfeit brand of Daflon (micronized purified flavonoid fraction) on the local market.
The product is used to treat patients suffering from chronic venous insufficiency (CVI) – varicose vein and venous circulation disorders (swollen legs, pain, early morning restless legs) and symptoms associated with acute haemorrhage, the GA-FDD said in a release yesterday.
The original product is sold in a white box with blue borders and containing sixty 500mg tablets that are further divided on four silver blister packs, each containing fifteen tablets, while the counterfeited/fake product is sold in a white box with no border and containing sixty 500mg tablets that are further divided on six silver with blue blister packs, each containing ten tablets. The manufacturers’ address of the original product is in France while that of the counterfeited/fake product manufacturers’ address is Turkey / Ukraine. See photo.
The official distributors of the original product are Massy Distributors Inc and ANSA McAl Trading Ltd. The release said that Inspectors of the GA-FDD have to date seized approximately twenty four boxes x 60 tablets x 500mg of the counterfeited/fake from several wholesaler bonds and retail pharmacies in Georgetown and in Region Three. Criminal proceeding are to be instituted against the importers of the fake/counterfeited Daflon.
The Department is calling on importers and distributors to with immediate effect surrender all the Fake/ Counterfeited Daflon associated with this mandatory recall to be destroyed. Failure to do same will leave the department with no alternative but to initiate legal proceedings against defaulters, the release said.