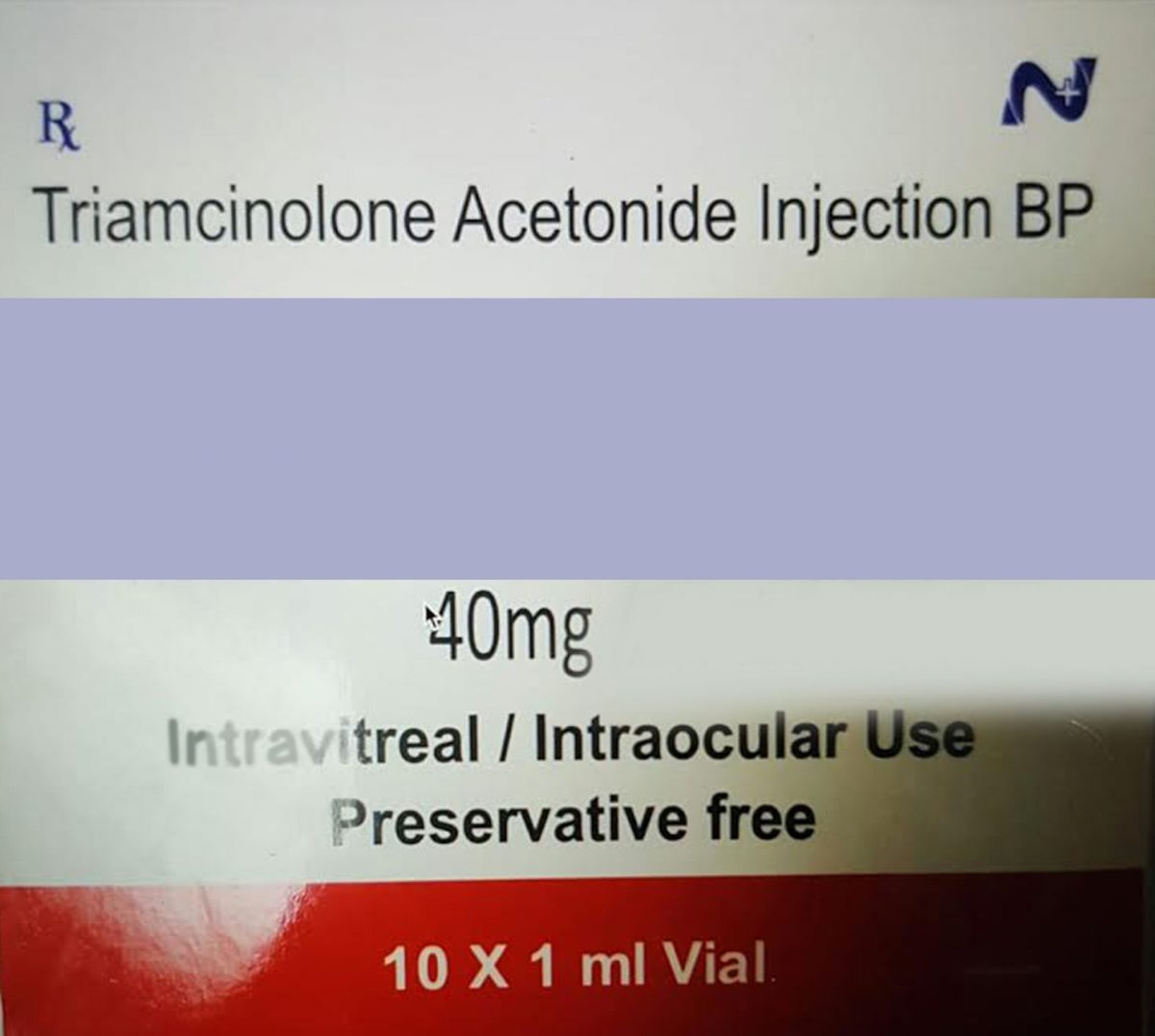
(Trinidad Guardian) A tainted eye injection—brought into Trinidad and Tobago via “illegitimate importation channels”—has left seven diabetic patients blind (either in both eyes or one). In the past four months, the patients have undergone eye removal surgery and are currently being outfitted with prosthetic glass eyes.
The Government recalled the imported injection which contains Triamcineclone Acetonide as its main ingredient back in July, but by then the damage was already done. The specific drug which contained the Triamcineclone Acetonide was a preparation from an India-based company.
In its July recall statement the Government said that the drug which contained Triamcineclone Acetonide BP was recalled due to “possible bacterial contamination” and that it was not registered in T&T.
“It would have had to be brought into the country via parallel illegitimate importation channels. The Chemistry, Food and Drug Division of the Ministry of Health has already seized stocks of this drug from the supplier,” the ministry stated.
Despite the issue of illegitimate importation, the supplier is still in business and the only action taken against the company was that it is no longer allowed to participate in government tenders.
There is conflicting information as to who is paying for the surgeries and subsequent treatment that the seven patients underwent since July.
Guardian Media was able to contact three of the affected patients but none wanted to speak about the matter.
One man said that he needed legal clearance before he could say anything about the matter. When asked if he had sought legal advice on how to proceed, he said he did not.
“I haven’t spoken to a lawyer,” he said.
When pressed as to why he would need to seek legal clearance, the patient said he could not talk about it.
“I cannot give any information,” he added.
Bacteria-laden drug
The bacteria-laden drug was administered by unwitting ophthalmologists in July and one doctor alerted the Ministry of Health when he realised the severity of the injection.
The retina specialist, who requested anonymity, spoke with Guardian Media last week Friday and said the patients were still undergoing follow-up treatment from the tainted injections.
Before this batch came in, the injections were in use by ophthalmologists in T&T for years.
This tainted batch, however, came in different packaging but was manufactured by the same India-based company and distributed by the same local company.
The injections were sold to ophthalmologists throughout the country and administered to patients with diabetic eye problems. Each injection cost patients between $500 and $1,000.
“It is traumatic to have to tell a patient that they could die if we do not remove their infected eyes,” the doctor said.
The doctor, who also worked intimately with the tainted batch of medication, said one day after administering a routine injection to one of his patients he received a call about an infection.
“It’s not unusual to have infections from the injections but it usually takes about four to five days before an infection shows. I was alarmed to see the state of the infection after only one day,” the doctor told Guardian Media.
He said he contacted a group of ophthalmologists who also used the drug and told them to stop immediately.
“We administer several of these injections a day. If we did not stop when we did, that count could have been between 40-50 patients,” he said.
The bacteria, the doctor said, was a “multi-drug resistant pseudomonas,” which meant it did not respond to regular treatments for bacterial infections and resisted antibiotic treatment.
“Nothing was killing the bacteria. We privately formed a group to try and figure out what to do to help the patients. Because the eye is so close to the brain we had to act fast,” the doctor said.
“It was a storm, everything happened so fast. Within a week all seven patients had to have surgery,” the doctor added.
The surgery to remove an eyeball is not done in public hospitals and the doctors had to have them done privately.
“We were able to have the doctors waive the costs because none of the patients should have to pay for this surgery,” the doctor said.
The retina specialist said the patients would consider legal action against the supplier once their treatment was completed.
“I don’t know the details on how it was registered in the country,” the doctor said.
Private clinic contacted
Guardian Media contacted the private clinic—based along the East-West Corridor—but was told that the matter was confidential and they could not provide information on “the amount of surgeries or the cost of such a surgery.”
“Something needs to be done. This shouldn’t be allowed to happen and scare people away from having these treatments,” the doctor said. “It is a rare occurrence.”
A second doctor associated with the private clinic, however, confirmed to Guardian Media that he was aware of the seven surgeries that resulted from the tainted drug.
He too could not confirm the cost of the procedures.
Guardian Media was told that the surgery cost depended on the type of anaesthetic that the patient needed and that with general anaesthesia the surgery would cost upwards of $10,000.
He said that he was not aware that the cost of the surgeries were “waived”.
“I know the clinic was paid,” he said.
Local distributor responds
In a brief interview last Thursday, the owner of the distribution company said he was aware of the tainted batch of injections and that the Ministry of Health had issued a recall of the drug.
Guardian Media was able to contact the owner of the local distribution company and he said that back in July when the drug was recalled he met with “the officials.”
“We complied with the ministry and provided all the documents that were requested,” he said.
However, he said he did not hear about the injections causing blindness or that patients had to have their eyes removed after being given the injections.
Guardian Media attempted to contact the owner again on Tuesday and Wednesday, and on Thursday visited the supplier. Security at the Central-based facility said he was unavailable. Guardian Media also visited and called again on Friday but was told that he was still unavailable.