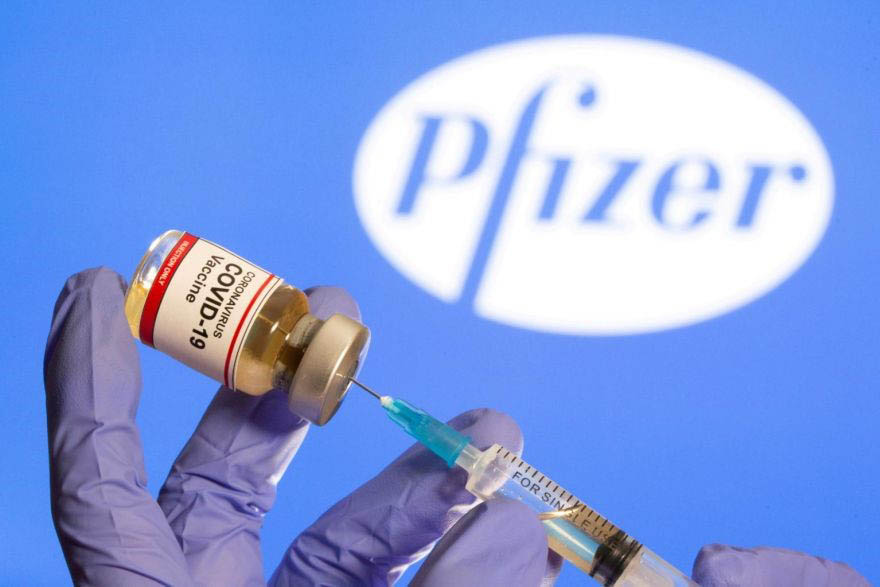
(Reuters) – A panel of outside advisers to the U.S. Food and Drug Administration yesterday voted overwhelmingly to endorse emergency use of Pfizer’s coronavirus vaccine, paving the way for the agency to authorize the shot for a nation that has lost more than 285,000 lives to COVID-19.
The FDA is widely expected to authorize emergency use in days. Distribution and inoculations in the United States are expected to begin almost immediately thereafter.
The committee voted 17-4 that the known benefits of the vaccine outweighed the risks of taking the shot for individuals 16 and older, with 1 member of the panel abstaining.
“This is a historic moment,” Eric Dickson, chief executive of UMass Memorial Health Care, who was not on the advisory panel, said after the vote. He called the vaccine from Pfizer and German partner BioNTech “the best solution to get us out of our current situation and help us save lives.”
Pfizer had asked that the two-dose vaccine be approved for use in people aged 16 to 85. Several advisory panel members discussed whether 16 and 17 year olds should be included in the recommendation. In the end, they voted on the question as put them by the FDA, which included 16 to 17 year olds.
It was not clear why four panelists voted against authorization but several mentioned they were not comfortable with including 16 and 17 year olds, arguing the risk to those individuals was low, and the evidence in the trial was scant.
“The final decision about whether to authorize the vaccine for emergency use will be made by FDA’s career officials,” the agency said in a statement.
The panel also discussed concerns raised by two reports of serious allergic reactions among vaccine recipients in Britain, and what to advise pregnant women, who were excluded in the study. Women of childbearing age comprise a large proportion of healthcare workers, who will be among those first in line to get the vaccine.
The FDA said during the panel meeting that there was not enough data to support or contradict use of the vaccine in pregnant women. The agency recommends they make the decision on their own with advice from their doctors.
Dr. Gregory Poland, a virologist from the Mayo Clinic in Rochester, Minnesota, who has previously served two terms on the FDA advisory panel, said he was surprised advisers did not voice more cautions about pregnant women.
The advisers also spent a major portion of the meeting discussing Pfizer’s plan to give volunteers who received a placebo in its trial the option to get the vaccine when they become eligible for it under recommendations set by state and local health officials.
Much of that focused on how an emergency authorization would affect the scientific integrity of Pfizer’s ongoing study and the way other vaccines are studied in the future. Pfizer believes it should offer the vaccine to people in the placebo group of its trial as they become eligible for the shot.
The concern, expressed by both the FDA and members of the advisory panel, is that telling trial participants who got placebo and who got the vaccine, called “unblinding,” will make it harder to continue collecting data on safety and effectiveness needed to win full FDA approval of the vaccine.
Documents prepared by the FDA ahead of the meeting did not point out any new safety or efficacy issues, raising optimism the United States would soon follow the UK and Canada authorizing the vaccine.
Britain’s health regulator on Wednesday advised some people with a history of anaphylaxis, an overreaction of the body’s immune system related to medicine or food, to avoid the getting the vaccine.
Dr. William Gruber, Pfizer’s senior vice president of vaccine clinical research and development, told the panel they “saw no serious allergic reactions to the vaccine” among the nearly 44,000 volunteers enrolled in the trial.
Nevertheless, an FDA official said the agency has asked Pfizer to add severe allergic reactions to their plans to study safety issues related to the vaccine once it’s authorized.
Pfizer and BioNTech last month said a two-dose regimen of the vaccine was 95% effective in preventing illness from COVID-19, and detailed data released in the agency’s documents showed the vaccine began showing some protection even before volunteers received a second dose.
The documents also disclosed data on safety including cases of Bell’s palsy among volunteers in the placebo and vaccine groups, though it said the cases in the trial occurred at the same rate as in the general population. Other reactions included fever, fatigue and chills.