By Khadidja Ba
Diabetic drug Ozempic which has triggered a global rush as it has been employed for weight loss is being dispensed in Guyana through medical spas, bottom-houses and a thriving suitcase trade but the authorities have warned that it has not been registered here for use.
Speaking with Stabroek News, an official from the Government Analyst-Food and Drug Department (GA-FDD), who declined to be named, confirmed that Ozempic in fact, is not registered in Guyana and therefore should not be imported into the country; much less be advertised for sale, distributed or administered.
The GA-FDD has warned routinely that any unregistered drugs/medicines in circulation will be seized.
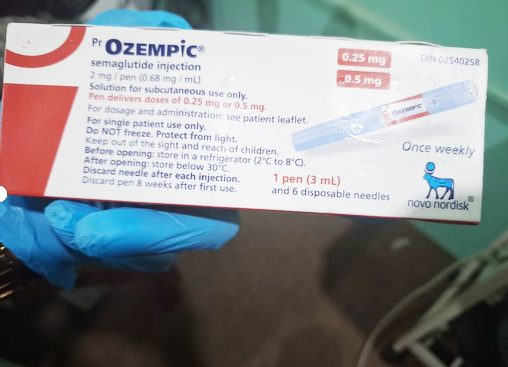
Speaking to Stabroek News, an entrepreneur admits to currently selling and administering the drug for the purposed of weight loss. The entrepreneur went on to state that it is US Federal Drug Administration (FDA)-approved and she is currently out of stock. She was not aware that the drug had to be registered locally, notwithstanding FDA approval. She also shared that “the medication is normally transported via cold storage, I would normally purchase from a licensed pharmaceutical distributor in Guyana and they would normally bring in the medication and I of course will take it from them. So they would know the ideal conditions with which to transport the medication. So as to ensure its efficacy at the end of the day because if it doesn’t reach a certain criteria then I don’t take it because it wouldn’t be effective enough.”
Acknowledging that this is a diabetic drug, the source related to SN that they “basically have a history that is a lab history of the health of the client and depending on those values, they decide what dosage to start them on. The majority of clients we start at the lowest dose so as to remain accountable and check their reactions in order to make sure that they get the best treatment and they are not consumed by their side effects. Those patients that are diabetic or pre diabetic benefit more from the medication in that it controls their blood sugar and they have that added benefit of weight loss.”
Other sources stated that one of the ways to purchase Ozempic in Guyana is through making contact with a Trinidadian who brings it back with him frequently from Trinidad through the CJIA, Timehri in his suitcase. Just recently, 24 pharmacies in Trinidad were under police probe for selling unregistered, fake and expired drugs which included unregistered and therefore illegally imported, Ozempic.
There are several local medical spas on social media advertising Ozempic for “weight loss”. Additionally, a well-known medical establishment has also shared with SN that they have discontinued their sale of Ozempic.
Prices locally for Ozempic varied significantly. The medical spas ranged from $100,000-$150,000 per dose, the medical establishment ranged around $200,000 per dose. To put these prices into perspective, each person would require a minimum of 4 doses (once weekly administered) depending on the assessment given by the medical professional. Ceasing Ozempic usage may lead to weight regain and decline in cardio-metabolic health.
According to Ozempic’s FDA Approval History, “Ozempic is a medication that has been FDA approved for the treatment of type 2 diabetes, and to reduce the risk of major cardiovascular events in patients with type 2 diabetes and known heart disease”. However, a great demand has come from those with the wherewithal to pay.
A version of Ozempic, Wegovy, also FDA-approved, is simply semaglutide (the generic medication found within Ozempic) with an increased dosage and repackaged. Ozempic/Semaglutide actually works as an appetite suppressant that slows down gastric emptying. In a way, the drug would be an appetite modulator of sorts, allowing one to consume less calories.
According to the Yale School of Medicine, the safety and effectiveness of this medicine has not been tested on persons who are already of a healthy weight and just want to lose a few pounds for a short term goal and then stopping it altogether.