(Trinidad Guardian) The Pan American Health Organisation (PAHO) team’s investigation into baby deaths at Neonatal Intensive Care Unit (NICU) in April has highlighted deficiencies in the operations at the Port-of-Spain General Hospital.
These range from inadequate staffing, the absence of personal sanitisation protocols and breaches in personal protective equipment (PPE) protocols, to the lack of procedures for the high-level disinfection of equipment.
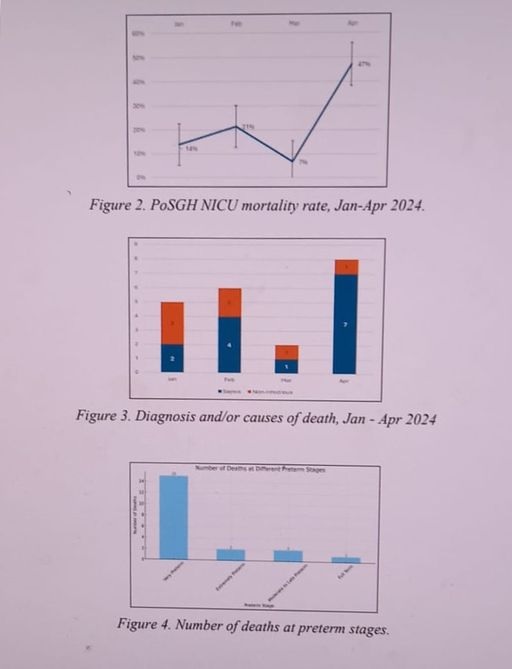
Overall, the PAHO team, which examined the clinical events at the NICU after seven newborns died between April 2-9, gave the specialty baby unit a 29 per cent rating, noting the highlighted areas showed a need for improved compliance monitoring and training.
“Use of multi-dose saline vials was observed which could lead to an increased risk of infections among patients. Use of single-dose saline vials only must be done,” the report said in one segment.
“Infections at surgical and procedural sites were linked to inconsistent or inadequate skin preparation practices, suggesting a need for standardised, pre-packaged 2% chlorhexidine solutions to ensure effective antisepsis. Use of pre-packaged 2% chlorhexidine skin preparation for preoperative skin prep, NICU procedures, and for caesarean sections must be done,” the report added.
PAHO also raised alarm that “there are no personnel responsible for Infection Prevention and Control at high levels within the hospital” and that “goals are not monitored and evaluated annually by hospital management.”
However, in its report submitted to the Health Ministry on June 21, it said epidemiological surveillance of infection was at 80 per cent even though the standardised definition of bloodstream infections (BSI) is not consistently used and standardised definition, including denominator data, is not collected.
“A significant strength identified in PoSGH is the presence of a dedicated IPC programme and a knowledgeable microbiology laboratory team capable of identifying pathogens.
“The commitment from both hospital administration and national authorities to enhance IPC measures was clearly evident and commendable,” the report added.
In addition to a series of hospital visits, the three-member PAHO team of experts, which was on the ground in Trinidad from April 22-26, conducted an extensive review of documents and interviews, “aimed to fortify the hospital and possible national efforts in preventing hospital-acquired infections with a particular focus on the NICU.”
The team included Professor of Paediatrics, Global Health and Epidemiology at George Washington University in Washington DC, Dr Nalini Singh MD, MPH; Clinical Microbiologist and head of Microbiology at Centro de Asistencia Medica Soriano in Uruguay, Dr Grisel Rodriguez, MD, PH; and Newborn Intensive Care Specialist and head of the Neonatal Care Intensive Unit at the Queen Elizabeth Hospital in Barbados, Dr Gillian Birchwood, MD.
Staff at the NICU had observed a rapid deterioration in the status of several newborns. Treatment was triggered for late-onset neonatal sepsis. Laboratory investigations revealed the presence of three different organisms – Serratia marcesens, ESBL Klebsiella pneumoniae, and Klebsiella aerogenes, all known to pose significant risks to vulnerable neonates(babies).
Health Minister Terrence Deyalsingh laid PAHO’s report in Parliament yesterday, noting the ministry is seeking urgent clarification on certain of its findings.
The report’s executive summary stated that PAHO’s technical assistance was requested by the ministry and that PAHO and the ministry agreed to conduct a review of the current IPC practices.
Findings: Room for Improvement
The report stated that an assessment of the national IPC programme was carried out on May 2, by applying the World Health Organisation (WHO) tool for periodic evaluation of infection prevention and control programmes through an electronic questionnaire by the national authorities.
Six WHO core components were evaluated: IPC programme, IPC guidelines, education and training in PCI, surveillance of Healthcare Acquired Infections, multimodal strategies, monitoring, auditing of PCI practices, feedback and control activities.
“Overall, the Infection Prevention and Control Assessment tool assessment reveals strengths in most of the six areas evaluated. However, there is room for improvement in development, dissemination, and implementation of national technical guidelines and in information analysed and timely feedback provided to all relevant stakeholders,” the report stated. “Addressing these areas is essential to enhance the effectiveness of the IPC programme at the national level,” the report added.
“At hospital level, compliance with Infection Prevention Control (IPC) assessment varied from 29 per cent to 80 per cent.”
The findings of PAHO’s Rapid Evaluation Tool also showed deficiency in the following areas:
Organisation compliance – 66 per cent
There are no personnel responsible for Infection Prevention and Control at high levels within the hospital; goals are not monitored and evaluated annually by hospital management.
However, epidemiological surveillance of infection was said to be at 80 per cent even though the standardised definition of bloodstream infections (BSI) is not consistently used and standardised definition, including denominator data, is not collected.
Additionally, PAHO highlighted that active surveillance for monthly Healthcare Acquired Infections/Blood Stream Infection (BSI) rates is not conducted and outbreak identification and number of outbreaks in the last year data are not readily reported.
Prevention & Control strategies -71 per cent
Institutional policies for central line insertion and maintenance in NICU aren’t used consistently.
Policies and procedures for prevention of surgical site wound infection in OB theatre isn’t done.
Antimicrobial stewardship programme isn’t implemented.
Hospital Environment & Sanitation – 64 per cent
Use of alcohol-based hand rub from hands-free dispensers is not being done.
Appropriate bed spacing (at least 1m between beds) is not present
Neonatal ICU – 29 per cent
Inadequate ratio of nursing professionals to patient ratio is present consistently.
Early breast milk feeds are not being instituted.
Policies and procedures for use of multidose medications on NICU need to be developed.
NICU unit dose medications are not prepared in sterile conditions by pharmacy. Sterile unit dose preparation of all NICU medications by pharmacy must be done.
There are no policies nor procedures for high-level disinfection for equipment such as laryngoscopes. Policies and procedures for high-level disinfection e.g. laryngoscopes must be developed.
Limited hands-free alcohol-based hand rub dispensers in NICU. More accessible hands-free alcohol-based hand rub dispensers need to be installed on NICU.
Lack of implementation of timely contact precautions for patients affected by multiple drug-resistant organisms. Contact precautions for patients harbouring MDROs must be promptly instituted.
Breaches in personal protective equipment (PPE) protocols observed in the NICU, indicating a need for improved compliance monitoring and training. Compliance monitoring of donning & doffing procedures for PPE and contact precautions on NICU using the WHO tool should be done.
Use of multi-dose saline vials was observed which could lead to an increased risk of infections among patients. Use of single-dose saline vials only must be done.
Infections at surgical and procedural sites were linked to inconsistent or inadequate skin preparation practices, suggesting a need for standardized, pre-packaged 2% chlorhexidine solutions to ensure effective antisepsis. Use of pre-packaged 2% chlorhexidine skin preparation for preoperative skin prep, NICU procedures, and for caesarean sections must be done.
Skin irritation and adverse reactions were frequently noted in neonates following the use of chlorhexidine baths, indicating a need for gentler, pH-neutral or mildly acidic cleansers.
Use individual pH neutral/mildly acidic gentle cleansers for neonates, discontinue chlorhexidine baths.
Preparation, storage and use of agents for used for skin prep prior to procedures is done in the pharmacy under unsatisfactory circumstances.
Ineffective practices: Cessation of shaving of surgical site.